Participants
Approximately 204 adults between 18-75 years of age
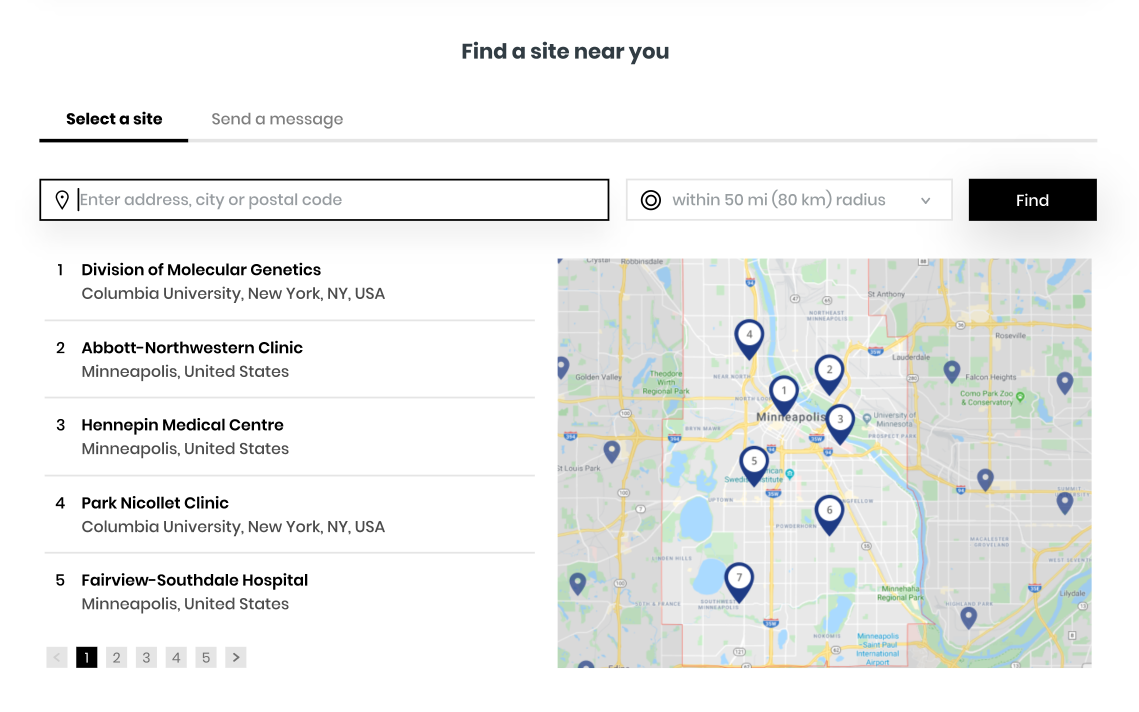
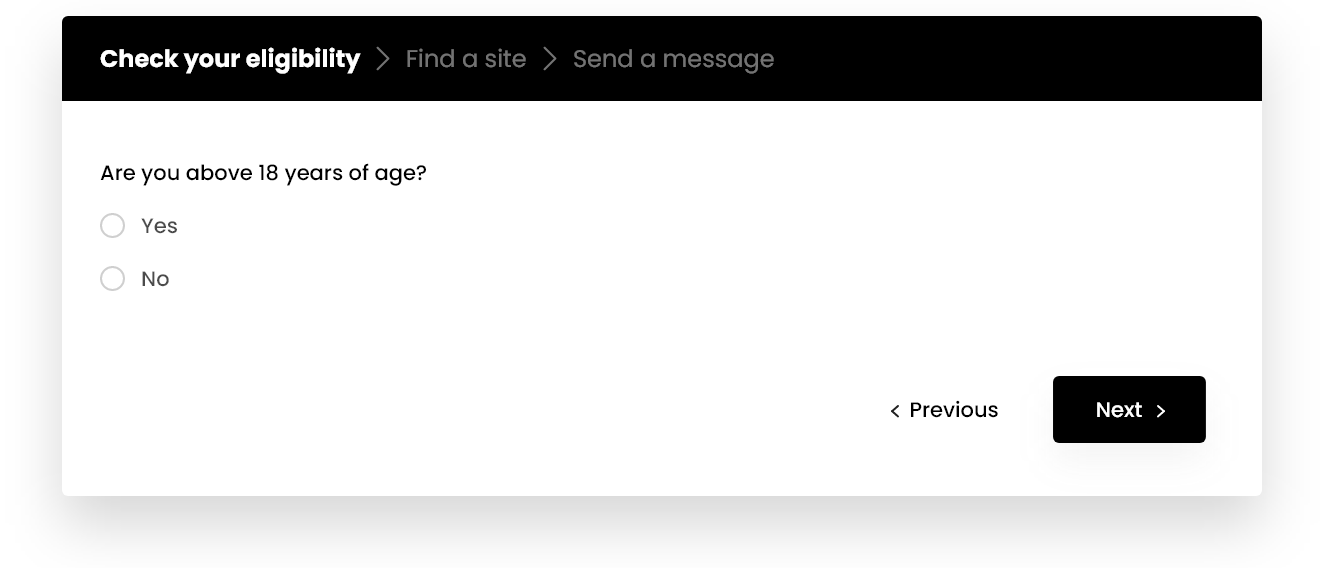
Check your eligibility now & get in touch with a study center
CHECK ELIGIBILITYAre you between the ages of 18-75?
Have you been diagnosed with ulcerative colitis (UC) for at least three (3) months?

Ulcerative colitis (UC) is a long-lasting condition that causes inflammation in the large intestine (also called the colon). The exact cause is unknown, but many believe it involves problems with the immune system.
Symptoms can vary depending on how much of the colon is infected and how severe the inflammation is. Individuals with moderate to severe UC may have symptoms such as abdominal pain (pain in the belly) and daily loose stools that may contain blood. Currently, there are no medications that can cure ulcerative colitis. There are treatments available to help manage the flare-ups (when symptoms get worse) and improve your quality of life.

In people with UC there is too much inflammation, which may be caused by a protein in your body called tumor necrosis factor alpha (TNFα). The study medication blocks this protein which may reduce the chronic inflammation and may lead to fewer UC symptoms.
The study medication is a tablet and is still being tested, so it's not available for general use yet. During this study, you have a 5 in 6 chance of receiving the study medication or 1 in 6 chance of receiving a placebo. A placebo looks like the medicine being tested and is also given in the same way but it does not have any medicine in it.
At your appointments several checks to monitor your health are included:

A physical exam, including checking your height, weight, and vital signs

Up to three (3) colonoscopies or sigmoidoscopies (a long flexible tube with a light and camera that is inserted into the rectum to view the entire inside of the colon) with biopsies also collected

An ECG (checks your heart's electrical activity using electrodes placed on your skin)


(if required)

Short, daily electronic questionnaires about number of stools and blood loss, quality of life (how you're feeling or daily activities) and medication usage
There is no cost to participate. All study-related exams, study-related medications and study-related medical care are provided. There is no insurance required to take part in this study. You may be compensated for time and travel.
Sanofi believes that everyone should have the opportunity to take part in clinical trials. It is important to include people who have been historically under-represented in clinical trials. Sanofi is committed to inclusivity in our studies.
If you are interested in learning more about these studies, we encourage you to complete this brief questionnaire to help determine if you may qualify to participate and be referred to a site recruiting in your area for further evaluation.
Version 1.0, 15Jan2025
Images property of Sanofi